Fingerprinting is commonly viewed as an infallible means of personal identification as forensic science has proven that the two human beings are not likely to have the same fingerprints. It has also been suggested that the fingerprints of any individual do not change throughout his/her lifetime with the exception of a significant injury that results in scaring on the finger(s).
There are three main types of fingerprints: visible prints, latent prints and impressed prints. Visible prints are also called patent prints and are usually left in some medium that reveals them to the naked eye. They can be formed when substances such as blood, dirt, ink or oils on the finger come into contact with a smooth surface a leave an impression that is visible without development.
Impressed prints are also called plastic prints and are indentations left in soft pliable surfaces, such as clay, wax, paint or other surface that will hold the impression. They are visible and can be viewed or photographed without development.
Latent prints usually refer to prints that are not apparent to the naked eye. They are formed from the sweat from sebaeceous glands on the body or water, salt, amino acids and oils contained in sweat. The various fluids create prints that must be developed before they can be seen or photographed. They can be made sufficiently visible by disting, fuming or chemical reagents.
Ridges and Pores activity
Introduction:
A friction or epidermal ridge is a raised portion of the skin on the fingers and toes. Impressions of fingerprints may be left behind on a surface by natural secretions of sweat from glands that are presnet in friction ridge skin.
WOOD GLUE method
AIM: To capture ridge and pore details of your finger using a polymer cast.
- Where are the pores found? Are they regularly spaced?
The pores are usually found against the fingerprint ridges. They are regularly spaced.
- Are the lines of your print equally spaced throughout?
Yes, they are. However, even though they look quite evenly spaced, but the spaces may be very equal.
Cyanoacrylate (Superglue) fuming method
Introduction:
The actual development of this chemical method of developing latent fingerprints is somewhat unclear as many agencies and countries claim credit for its discovery. What is generally accepted is that the method came about between 1977 and 1978. It has since been used as an effective technique in many crime laboratories throughout the world.
The basic concept behind all chemical techniques is to apply something that will chemically react with one or more of the constituent chemicals of latent fingerprints. The product of the chemical reaction will render the fingerprints visible and allow the print to be photographed so that identifications can be made.
Superglues typically contain methylcyanoacrylate or ethylcyanoacrylate. They react with traces of amino acids, fatty acids and proteins in the latent fingerprint and the moisture in the air to produce a visible, sticky white substance along the ridges of the fingerprint. The resulting image is then photographed so that comparisons can be made with other fingerprints that are collected.
To enable such a reaction to take place, the cyanoacrylate must be in its gaseous form. Hence, investigators tend to use a fume box to hold the chemicals and objects in an enclosed space. Heat is then applied to allow the superglue to vapourise. If there are any latent fingerprints on the object, the exposure and humidity in the atmosphere will be sufficient to enable the reaction to take place. The actual amount of time required for such a reaction to take place depends on several factors: concentration of cyanoacrylate fumes, humidity levels and size of fume box etc. It is often required to monitor the reaction as it is almost impossible to predict the actual amount of time required for the prints to develop.
AIM: To develop latent prints on a non-porus surface using superglue fuming method.
- If the contrast of the white print against the black background is still too faint for a good detailed photograph to be caputured, what could be done to enhance the fingerprint?
Flourscent dye can be applied onto the print and then placed under UV light to make the print visible.
iodine fuming method
Introduction:
The iodine fuming method has historically been recognized as one of the earliest techniques used by investigators for developing latent prints. Prior to the introduction of chemical methods such as using ninhydrin and silver nitrate, iodine fuming was a preferred and recommended method to be used on paper products.
While frequently categorised as ‘chemical technique’, the development of latent prints with iodine fumes is not a chemical process but a physical one. Chemical reactions produce new substances and will result in changes of the properties of the constituents of the latent print. Eg. Silver nitrate reacts with the chloride from sodium chloride to form a white precipitate – this creates white lines that make up the latent print.
However, in iodine fuming, natural body fats and oils in the larent print temporarily absorb the iodine vapours. This results in a change in colour, often from colourless to dark brown. This temporary change fades with time as the iodine that is absorb will eventually dissipate into the atmosphere. The colour change can be made permanent by the application of certain materials but the developed latent print can usually be photographed at the greatest inensity of colour change and then allowed to fade.
One advantage of using iodine fuming is that it is an essentially non-destructive technique. No permanent or chemical change has taken place after the print develops. The latent can therefore be further processed using other methods. Additional tests for the constituents of the latent can also be conducted after the print is developed.
AIM: To develop latent prints on a porus surface using iodine fuming method.
- What are the possible substances that may be used to render the prints more permanent?
A water and starch solution.
- Why does the prints disappear?
Powder dusting method
Introduction:
The most commonly known method for developing latent prints would probably be the powder dusting method. A variety of powders are used in dusting for prints. Many of these powders contain aluminium or carbon. The finely crushed powder is gently applied to a surface and the minutes particles of powder cling to the latent residue, making it visible. These prints are then lifted using adhesive tape. For dusting to work, the surface that is being dusted must be completely dry and relatively free of other contamination.
The principle behind dusting is simple. Oils and perspiration form the common residue on fingers that get transferred when a print is made. When the powder is applied to the surface with the print, it sticks to the oils and brings out the ridge patterns.
Dusting is ideal on wood, metal, glass, plastics and tiles. It is less than ideal on paper, cardboard and leather. Powders vary in colour, stickiness, photographic and magnetic qualities. The best colour to to use is one in shart contrast to the surface colour. For example, a white or grey powder works best on a dark surface and a black powder works best on a white or colourless surface. In multicolour situations (such as a magazine cover) it is best to use a fluorescent powder. When the dusted object is exposed to ultraviolet light, the powder will glow, making the print show up regardless of the background colour.
Aim: To develop latent prints using powder dusting method
- What is magnetic powder dusting and how does it work?
Magnetic powder dusting is the used of magnetic powder to reveal fingerprints. Magnetic powder dusting does not require a brush, unlike regular dusting. By using a magnetic wand, the powder would be attracted to the wand and as the wand moves, thus, only the powder alone would move over the print.
Classification and identification of fingerprints
Introduction:
It is not clear which civilisation first utilised fingerprints as a form of identification. Evidence suggests that ancient Egyptians and Chinese create fingerprints on clay objects and official documents as a form of identification. There are however no known documents that describe how identification was conducted in ancient times.
Modern development of fingerprinting was mostly documented from 1858, when Sir William Herschel, British Adminstrator in District in India, required fingerprint and signatures on civil contracts. In 1892, Sir Francis Galton, a British Anthropologist and cousin to Charles Darwin, published the first book on fingerprints. He identified the individuality and uniqueness of fingerprints. In 1901, the first fingerprint bureau was eastablished in Scotland Yard. The system of classification was developed by Sir Edward Henry and is still the basis on which modern automated systems work to compare and identify fingerprints.
The three basic fingerprint patterns are Whorl, Arch and Loop. There are more complex classification systems that further break down the pattern to plain arches or tented arches. Loops may be radial or ulnar. Whorls also have more detailed classifications.
According to the fingerprint database in the US:
- 60-65% are classified as loops
- 30 – 35% are classified as whorls
- 5% are classified as arches
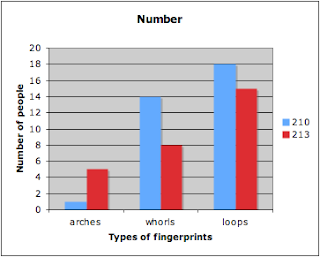
Number:
Finger Pattern | 210 | 213 |
arches | 1 | 5 |
whorls | 14 | 8 |
loops | 18 | 15 |
Graph
Percentage:
Finger Pattern | 210 | 213 |
arches | 3.03 | 17.86 |
whorls | 42.42 | 28.57 |
loops | 54.55 | 53.57 |
| 100 | 100 |